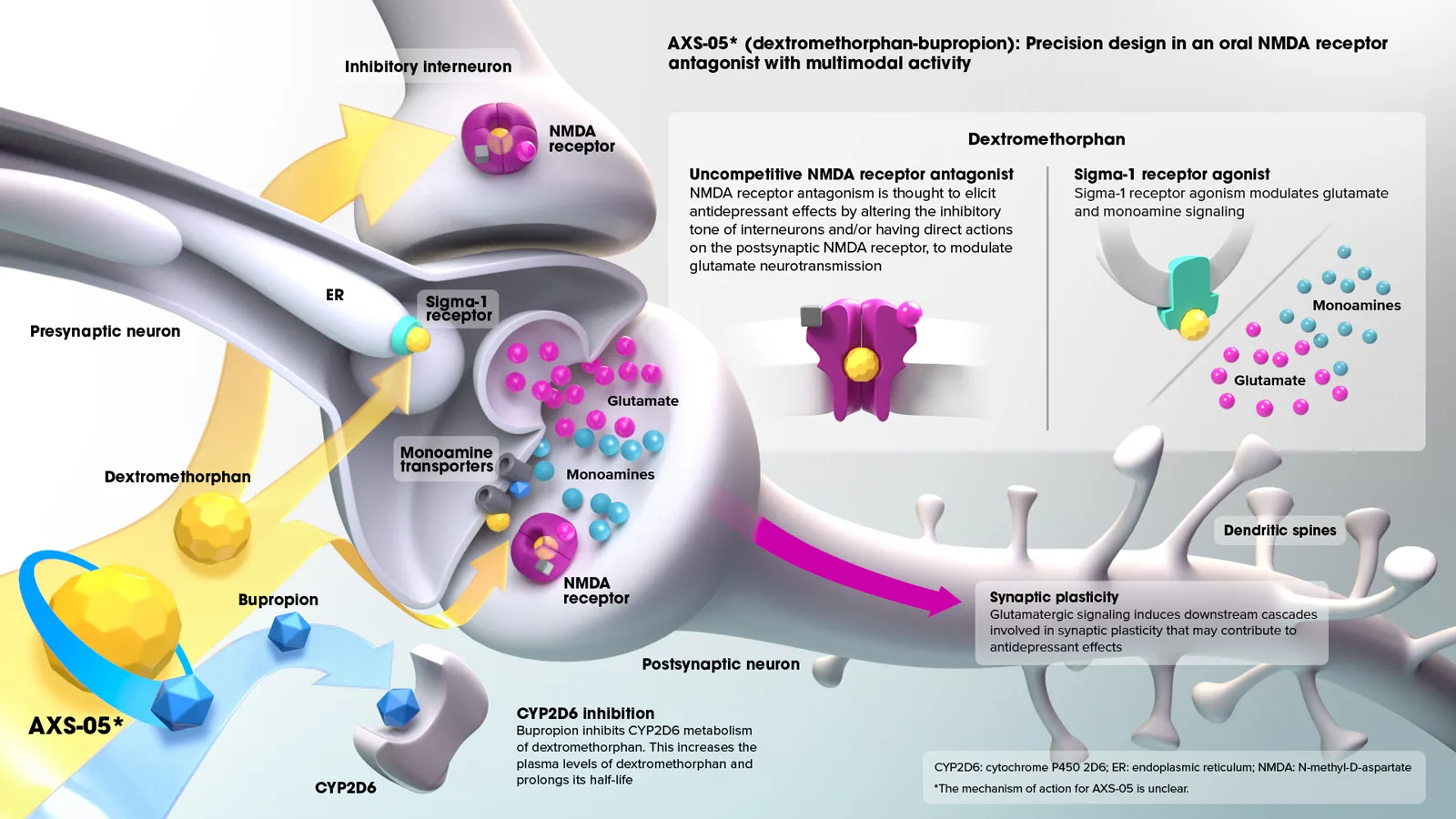
AXS-05 is a novel, oral, investigational NMDA receptor antagonist with multimodal activity being developed for the treatment of central nervous system (CNS) conditions. AXS-05 consists of a proprietary formulation and dose of dextromethorphan (DM) and bupropion.
-
The dextromethorphan component of AXS-05 is an antagonist of the NMDA receptor, an ionotropic glutamate receptor, and a sigma-1 receptor agonist
- These actions are thought to modulate glutamatergic neurotransmission
-
The bupropion component of AXS-05 serves primarily to increase the bioavailability of dextromethorphan, and is a norepinephrine and dopamine reuptake inhibitor
The safety and effectiveness of AXS-05 in Alzheimer’s disease agitation and smoking cessation have not been established and it is not approved by the FDA for these indications.
clinical and regulatory
AXS-05 is currently being developed for the treatment of Alzheimer’s disease agitation and smoking cessation. We have received Breakthrough Therapy designation from the FDA for Alzheimer’s disease agitation.